A Gallery of Selected Data Figures
Research in the Cooke lab was directed toward two broad questions:
•The physiology of rhythmic neural impulse burst formation, and
•The roles of electrical activity and calcium in the control of neurohormone secretion. Important connections between these are the crucial role of voltage-gated calcium currents in both, and the presence and importance of bursting for secretion in neurosecretory cells.
The research focused on observations from single neurons or small groups, as in the 9-celled cardiac ganglion activating the heart of crabs and lobsters. These animals offer neurons that are large enough to survive penetration by microelectrodes and that are individually identifiable from animal to animal. The X-organ-sinus gland of certain crabs provided secretory terminals large enough to sustain recording with microelectrodes before the era of patch-clamping. Isolated cardiac ganglia and isolated X-organ-sinus gland preparations remain viable and active for hours under perfusion with simple salines.
The tools and techniques available for neuroscience research improved and expanded dramatically during the 50 years I was in the lab. Microelectrodes had been in use less than a decade and appropriate electronics weren’t commercially available. Tektronix began selling oscilloscopes with a linear (rather than logarithmic) time base in the late 1950’s. With the benefit of consistent NSF and NIH funding, the lab was able to purchase advanced equipment as it became available.
The people who joined the lab are the most important element in the original and productive contributions of the lab to cellular neurobiology. They were dedicated, inventive, skillful, and hard-working collaborators and colleagues who participated in and often initiated the projects undertaken. For full references to the material represented in the figures shown, please see my Curriculum Vitae.
I. Physiology and neurohormonal control of crustacean hearts; pacemaker mechanisms.
Review: Cooke, I.M. (2002) Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol. Bull. 202: 108-136.
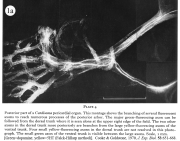 |
 |
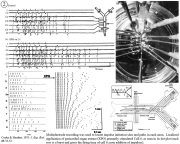 |
 |
 |
 |
 |
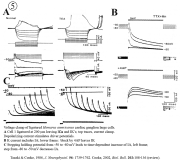
II. Control of neurohormone secretion: Intracellular recording from secretory terminals.
Reviews: Cooke, I., and E. Stuenkel (1985) Electrophysiology of invertebrate neurosecretory cells. In: A. M. Poisner and J. Trifaro (Eds.), The Electrophysiology of the Secretory Cell. (New York, Elsevier), pp. 115-164.
Stuenkel and Cooke (1988) Electrophysiological characteristics of peptidergic nerve terminals correlated with secretion. In: D. Ganten and D. Pfaff (Eds.), Current Topics in Neuroendocrinology. (Heidelberg, Springer-Verlag), Vol. 9, pp. 123-150.
 |
 |
 |
 |
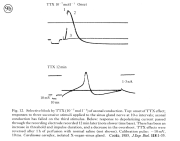 |
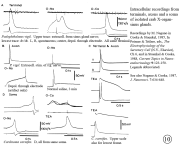 |
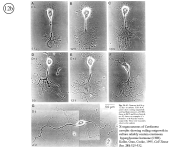
III. Characterization of dissociated crustacean neurons cultured in defined medium.
Review: Cooke, I.M. (1999) Physiology of mature crustacean neurosecretory neurons in culture. In: L.W. Haynes (Ed.), The Neuron in Tissue Culture. (New York, Wiley & Sons), Ch. 18, pp. 261-277.
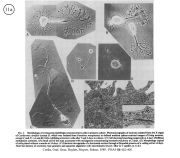 |
 |
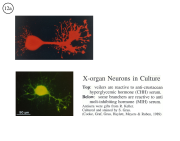 |
 |
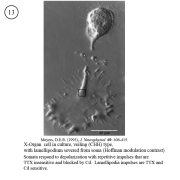 |
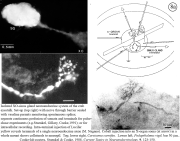
IV. Isolation of physiologically functional neurosecretory terminals.
 |
 |
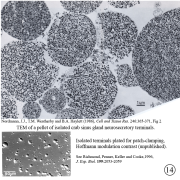
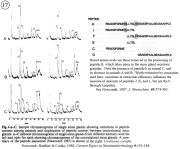
V. Neurohormone processing in secretory terminals.
Review: Newcomb, R.W., D.K. Hartline, and I.M. Cooke (1988), Changes in information content with physiological history in peptidergic secretory systems. In: D. Ganten and D. Pfaff (Eds.), Current Topics in Neuroendocrinology. (Heidelberg, Springer-Verlag), Vol. 9, pp. 151-183.
 |
 |

